- Human iPSC Stem Cell Line Development
- iPSC and MSC Stem Cell Line Banking
- Development and manufacturing of human/mammalian cell lines in stirred tank bioreactors (multiple 0.25 L scale (DASbox), cultivation in suspension or adherent using microcarriers or agitated packed bed technology)
- Product Purification: Filtration, Tangential Flow Filtration (TFF), Chromatography
- Upstream and Downstream Process and Product Development
- GMP services are currently in implementation, please contact us for timelines
- Pre-clinical and early clinical supplies of human iPSC, MSC and MSC-EV preparations
We are unlocking the full potential of regenerative medicine through our innovative extracellular vesicles (EVs) technology.
Our EV products are inspired by the body’s own healing mechanisms, capturing the therapeutic power of stem cells, without the hurdles and limitations of traditional cell-based therapies.
With our EV therapeutics we aim to address critical unmet medical needs, by ensuring reproducibility, scalability and safety.
Therapeutic Pipeline
Organ | Indication | Discovery | Pre-Clinical | Clinical |
skin | (Diabetic) Wound Treatment | ✓ | ✓ | |
heart | Cardioprotection / Reperfusion Injury | ✓ |
| |
undisclosed | Several indications are currently under investigation | ✓ |
|
Products
Our core values safety, quality, efficacy and scalability guide the development of stem cell products, enabling novel therapies.

For fast and successful clinical translation, products and processes are designed in a GMP-ready manner. Ethical and legal requirements are fully met for GMP-grade cell lines. GMP cleanroom resources are currently being established.
In addition to orthogonal analytical product characterization, we actively collaborate with clinical research centers and have collected compelling in vitro and in vivo data. These results demonstrate consistency in quality and biological functionality of our products.
We are ready to take the next step.
MSC
Mesenchymal Stem (Stromal) Cells
- Telomerized MSCs (MSC/TERT)*:
- Extended lifespan (>50 PD)
- Preserved MSC morphology and potential
- Eliminated donor variability
- Tiered cell banking (Master/Working Cell Banks)
GMP-ready (fully documented)
MSCale™
- Bioreactor-based cell expansion
- Use of GMP-grade materials
- Reproducible Processes and Product quality
- Conventional 2D Expansion and/or
- Scalable Production in Stirred Tank Bioreactor using Microcarriers
Panel of Quality Control methods
*Under commercial license agreement with Evercyte GmbH
EV
Extracellular vesicles (Exosomes)
- Native Extracellular Vesicles are harvested from stem cells (MSC)
- Patented continuous perfusion process in stirred tank bioreactors
- High biological activity of EVs is preserved by a gentle and effective purification process
- Developed for fast transfer into GMP facilities:
- Use of GMP-grade materials only
- Serum-, human Platelet Lysate (hPL)- and animal-free
- Free from antibiotics and Phenol Red
- Reproducible process and consistent product quality
- Scalable cultivation and purification processes
- R&D-grade EV preparations are available for testing
- Controlled and standardized processing including final sterile filtration
- Extensive analytical and bioactivity data available, e.g.:
- Anti-Inflammatory
- Anti-Fibrotic
- Wound Healing
- Consistent Marker Profiles and microRNA patterns
- Pre-clinical Testing (ongoing)
- We are currently testing EV preparations for several indications in Regenerative Medicine (RM)
- Please contact us in case you would like to learn more
iPSC
Induced Pluoriputent Stem Cells
- Cells for reprogramming isolated from human urine, blood or other sources
- Virus-free reprogramming and selection of suitable clones
- Cell banking and quality control (QC) of the selected iPS cell line(s)
- Scalable Production of iPS cell mass in Stirred Tank Bioreactors
- Developed for fast transfer into GMP:
- Use of GMP-grade materials
- Serum-, human Platelet Lysate (hPL)- and animal-free
- Free from antibiotics
- Reproducible cell expansion processes and product quality
- QC methods established (R&D, GMP in preparation)
- Differentiation processes (bioreactor-based) for selected tissue cells are in development
Technology Platform
Phoenestra has established GMP-aligned processes for both cell- and vesicle-based products derived from human Mesenchymal Stem Cells (MSCs) and induced Pluripotent Stem Cells (iPSCs), driving the development of novel therapeutics.
Our strong commitment to high-end bioprocessing ensures productive, reproducible and scalable process performance.
Clinical translation was considered by the early establishment of strategies for CMC Control, robust Safety and Containment protocols, and adherence to Regulatory Compliance guidelines.
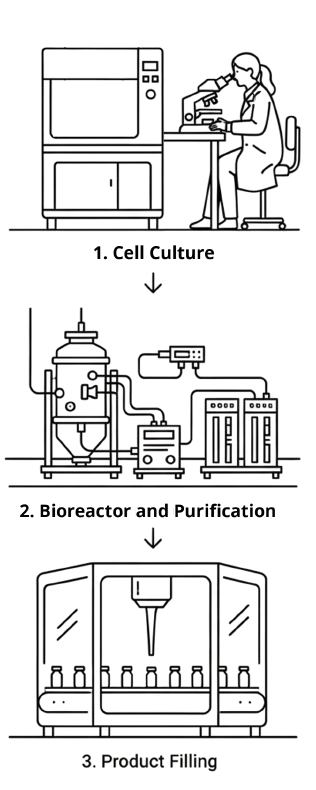
Services
Phoenestra offers R&D and GMP services designed to fit your specific project needs.
Cell Line and Process Development
Extracellular Vesicle Characterization
- Particle concentration
- Particle size
- Marker phenotype (tetraspannins: CD9, CD63, CD81; MSC marker: CD73, CD90, CD105; immunomodulatory markers: CD142, HLA-G, beta2-microglobulin, PD-L1; lipid content: MemGlow™)
- Protein concentration
- CD73 activity
- Biological Activity Assays: anti-Inflammation, anti-Fibrosis (both Evercyte), Wound-healing
- miRNA profiling (TAmiRNA)
- Sterility, virus testing, endotoxin
Cell Characterization
- Growth and morphology
- Surface marker analysis
- Differentiation
- STR profiling, karyotyping
- RNA expression analysis (TAmiRNA)
- Particulate and Biochemical Analytics, Bioassays, Disease Models
- Sterility / microbiological purity incl. mycoplasma, virus testing, endotoxin
Partners / Network
Publications / Resources
Get in touch.
For enquiries regarding our technology platforms, products and services, please contact
For any general inquiries or in case you are interested to join our team, please contact