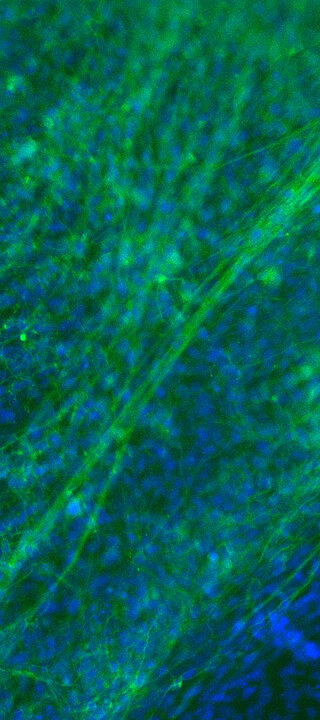
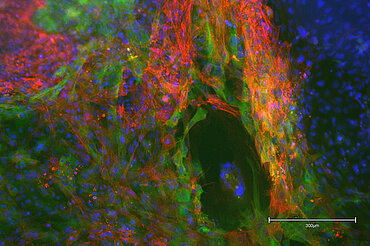
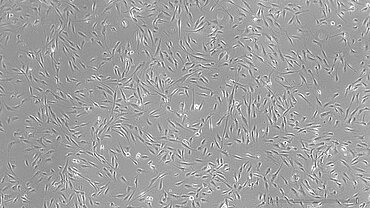
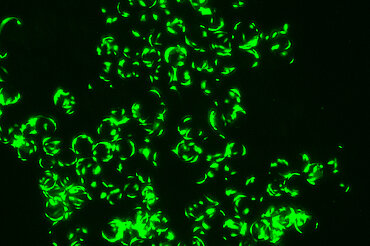
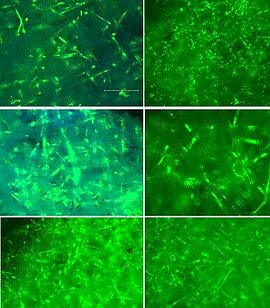
Our patented approach to use cells isolated from urine samples provides a non-invasive source for reprogramming somatic cells to induced pluripotent stem cells (iPSCs) from any healthy individual or specific patient, respectively. Selected iPS clones undergo a rigorous screening and characterization approach. Our iPS research cell banks fulfil all requirements for manufacturing of GMP-grade cell banks.
Mesenchymal Stem/Stromal Cells (MSC)
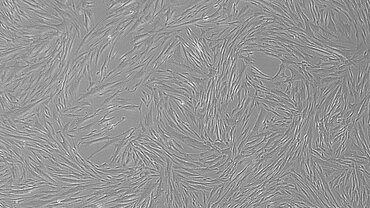
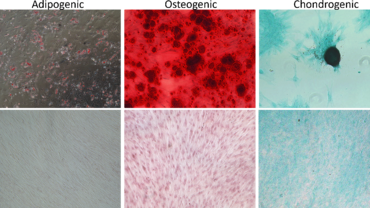
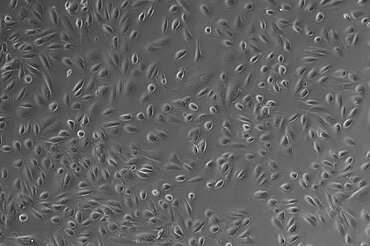
We have access to Evercyte´s industry-leading telomerized MSC lines derived e.g., from Bone Marrow, Adipose Tissue, Umbilical Cord, Wharton´s Jelly, Chorionic Plate or Placenta. In contrast to primary MSCs, they maintain their phenotype and consistent growth properties over many generations. Depending on the selected donor and tissue of origin, these MSC lines and their extracellular vesicle preparations display promising biological activities for Regenerative Medicine applications.
Scalable Bioprocessing
The cell and gene therapy (CGT) field is currently still suffering from technological issues and rather low technological maturity levels. Phoenestra is committed to contribute to bridging the existing “industrialization gap” in CGT manufacturing technologies and products. This gap consists of technological pain points and sometimes prohibitive cost of manufacturing (CoM). Phoenestra is developing scalable processes including Control Strategies for cell- and vesicle-based products. The combination of stable, high quality cell lines and scalable bioprocesses with CMC Control Strategies will help bringing much needed products and therapies forward and, ultimately, help providing better access of patients in need to these innovative therapeutic modalities.
Cultivation of Cells in Bioreactor Systems
Phoenestra is committed to scalable process technologies which enable us and our partners to move into pre-clinical and clinical testing on a fast track. We are using scalable stirred tank bioreactors for producing cell and vesicle products in significant quantities with consistent quality profiles. We are largely supplier agnostic and develop the best setup and cultivation mode:
- microcarrier, suspension/aggregate or spheroid
- fed-batch or perfusion
Interested? Contact us!
Isolation of Cells and Cell-derived Products
Depending on the cultivation mode, cells or extracellular vesicles are harvested directly from the culture broth or perfusate. Adherent cells are detached from microcarriers e.g., by a combination of enzymatic and mechanical treatments.
The respective product is then concentrated and washed by tangential flow filtration (TFF) before further downstream processing of cells or cell-derived products. Depending on the product targeted, different membrane or hollow fiber cut-offs are used and combined with filtration steps.
Interested? Contact us!
Processing of Cells
Depending on the purpose of the cultivated cells, cells are singularized, washed and formulated before storage and shipment or further processing. In addition, we are working on bioreactor-based differentiation methods. Purification steps to remove specific cell types after differentiation can be developed or implemented as needed.
Also, Phoenestra is actively pursuing innovative vesicular modalities and associated manufacturing technologies and processes, including cell extrusion technologies for the generation of nanovesicle preparations. Another approach that we pursue is the preparative enucleation of cells for therapeutic purposes.
Interested? Contact us!
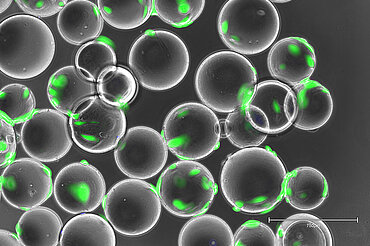
Extracellular Vesicle Technology - EVscale™
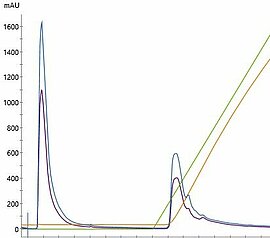
Extracellular vesicles (EV), or their sub-fraction called exosomes, hold great promises in Regenerative Medicine and beyond. Phoenestra has established a compehensive EV platform technology - EVscale™ - which is adaptable to different producer cell lines and EV / exosome target product profiles. EVscale™ - Extracellular Vesicles at scale comprise a proprietary perfusion bioreactor setup, depth filtration and membrane separation (TFF) methods as well as chromatography steps as needed. For analytical characterization, a range of standard and advanced analytical methods has been established which are performed in house or in collaboration within our network. Using functional biological assays, protein profiling and RNA sequencing we are able to assess the quality of EV derived from cultivations with different cell lines (e.g., MSC/TERT from different tissues) and between cultivation or preparations, respectively.
Interested? Contact us!
Products
Based on our stem cell technology platforms, Phoenestra provides access to a wide variety of promising cell-based product candidates for R&D, drug discovery and therapeutic applications. From the start we have the requirements of the final product in mind.
The selection of donors and donor materials is performed with all safety, ethical and legal requirements in place. For iPS cell-derived products, Phoenestra is currently establishing an end-to-end approach from urine sampling to release of GMP-grade products. For MSC-based products, we provide a one-stop-shop concept in collaboration with Evercyte (stable cell lines, potency assays) and TAmiRNA, the expert company in RNA profiling and biomarker development.
Interested? Please contact us for more details!
Products available and in preparation:
- Xeno-free iPS cell lines
- Telomerized MSC (TERT) lines (GMP-grade, licensed from Evercyte)
- Bulk iPS cells and TERT/MSCs from controlled bioreactor cultivations
- Extracellular Vesicle preparations from various cell lines / types
- Engineered “universal” stem cell lines (in development)
- iPS cell-derived (pre-)differentiated cells (in development)
- Processed cells (in development)
Services
Phoenestra offers capabilities and resources also on a fee-for-service basis and dependent on your specific project needs. Please contact us for a discussion of options and opportunities for R&D work or a collaboration regarding GMP manufacturing services. Or simply send us your “Request for Proposal (RfP)”
R&D
- Cell line development (xeno-free iPS cell lines)
- Cell banking (iPSC, MSC)
- Process and product development
- Process and product characterization
- EVscaleTM - Extracellular Vesicles at scale from different GMP-ready MSC/TERT cell lines at quantities up to 1x1013 EV per batch (TFF retentate)
GMP (please contact us for timelines)
- GMP Cell banking
- Pre-clinical and early clinical supplies